Materials: Medical TPEs Formulated Without Animal Derivatives
Avient’s new Versafle TPE grades offer a wide range of durometers for a variety of medical device applications.
Eight new grades have been added to the healthcare portfolio of Versaflex TPEs from Avient Corp. (formerly PolyOne), Avon Lake, Ohio. The injection-moldable Versaflex HC 3810 series is formulated without animal derivatives and offers a wide range of durometers to serve a variety of medical device applications.
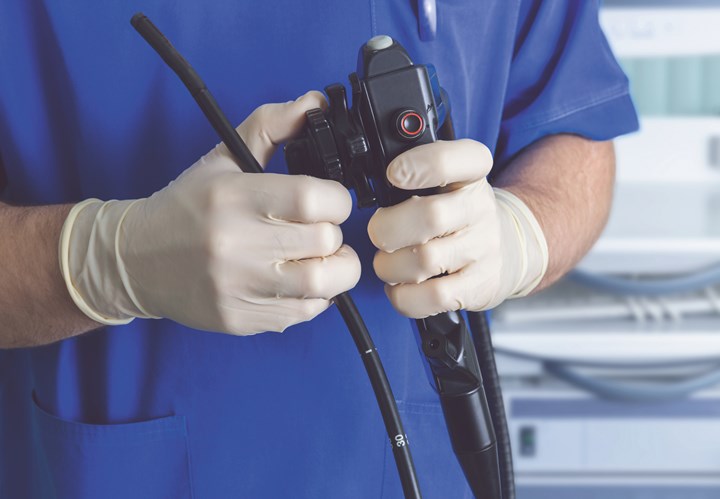
Commercially available in North America, the new specialty Versaflex grades are translucent and are said to offer excellent colorability. They are well suited for overmolding on PP. Example applications for 20-50 Shore A grades include ergonomic grips, strain relief components, handles, gaskets, and seals. Grades in the 60-90 Shore A durometer range can be used for IV connector caps, diagnostic testing devices, endoscopic caps, and similar applications.
The materials in this series can be used with gamma and autoclave sterilization methods. All eight grades are also ISO 10993-4 and -5, REACH SVHC, and RoHS compliant.
Related Content
-
Impacts of Auto’s Switch to Sustainability
Of all the trends you can see at NPE2024, this one is BIG. Not only is the auto industry transitioning to electrification but there are concerted efforts to modify the materials used, especially polymers, for interior applications.
-
BMW Group Vehicle to Adopt 3D Printed Center Console
A vehicle coming to market in 2027 will include a center console carrier manufactured through polymer robot-based large-format additive manufacturing (LFAM).
-
How to Optimize Injection Molding of PHA and PHA/PLA Blends
Here are processing guidelines aimed at both getting the PHA resin into the process without degrading it, and reducing residence time at melt temperatures.



