For Honeywell’s Aclar Edge Bottles, “The Pie Is Much Bigger Than We Thought”
High-moisture-barrier Aclar Edge bottles for liquid drugs are nearing commercialization, and much broader market opportunities are emerging.
In a Starting Up report last August, announced the debut of its Aclar Edge bottles offering glass-like clarity, high purity and chemical resistance, and moisture barrier on par with glass. This was the first application of Honeywell’s Aclar PCTFE resin in blow molding, rather than film. The bottles, extrusion blow molded for Honeywell by outside partners, consist of PCTFE, adhesive, and an outer structural layer of PET or polycarbonate.
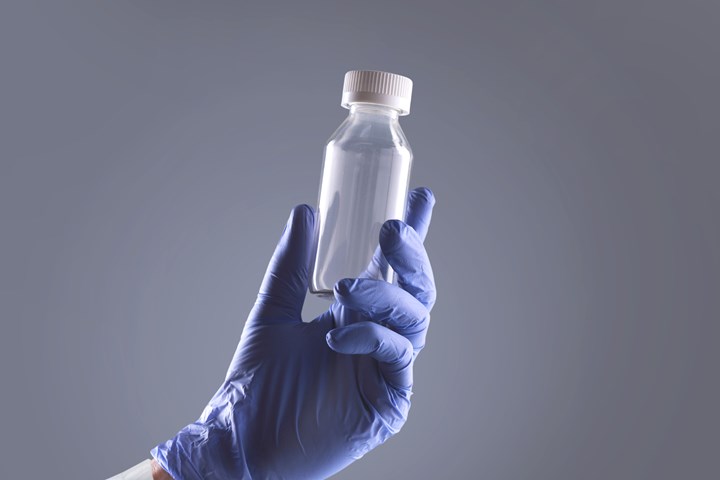
Honeywell’s Aclar Edge barrier bottles are glass-clear, but amber versions are in development.
Since our last report, Honeywell PMT has sent out thousands of samples for applications in pharmaceutical and nutritional oral liquids, according to Kori Anderson, general manager of Honeywell’s healthcare packaging ´óÏó´«Ã½. She tells Plastics Technology that a key step in customer evaluation of the bottles as glass replacements is drug stability studies of six to 12 months. “Commercial opportunities are now on stream for implementation this calendar year,” she says.
Market exploration have revealed some surprises, Anderson adds. One is opportunities for Aclar Edge bottles with some high-pH drug formulations that are aggressive against glass, causing delamination and formation of particulates. Another is the desire of customers for customized rather than just stock shapes. In addition, Honeywell has encountered high demand for amber-colored as well as glass-clear bottles and is trialling them now. “The pie is much bigger than we originally thought,” she concludes.
As a result, Honeywell is looking to expand bottle sourcing beyond the U.S. to South America and Europe. The company’s initial offering was a 120-ml stock bottle, but sizes from 30 to 500 ml are due out soon. In addition, Honeywell is actively developing smaller sizes for vaccine dispensing, and is therefore exploring alternative production methods such as injection-blow molding. Furthermore, “While we have been overwhelmed by interest in human oral liquids, there is definite interest in animal health applications,” notes Anderson. Some of those formulations contain aggressive solvents and the containers come in larger sizes, so breakage in the field has a larger economic impact—two more opportunities for chemically resistant and breakage-resistant Aclar Edge bottles.
Related Content
-
Medical Tubing: Use Simulation to Troubleshoot, Optimize Processing & Dies
Extrusion simulations can be useful in anticipating issues and running “what-if” scenarios to size extruders and design dies for extrusion projects. It should be used at early stages of any project to avoid trial and error and remaking tooling.
-
Medical and Molding Elite
When Jeff Smith received a notice evicting his promising ´óÏó´«Ã½ out of his house, it could have been the end of Elite Biomedical Solutions’ and Elite Precision Plastics’ stories before they really got started, instead it was just the beginning.
-
What to Look for in High-Speed Automation for Pipette Production
Automation is a must-have for molders of pipettes. Make sure your supplier provides assurances of throughput and output, manpower utilization, floor space consumption and payback period.



